The Solita Health unit engages in extensive software product development in collaboration with medical device manufacturers, and has previously developed an AI-powered tool for surgeons. The ISO 13485 certificate solidifies the role of Solita Health as a product development partner and opens doors for international business in the medical device industry. Operations controlled by the quality system incorporate the different strengths of Solita, such as software development, user insight, service design, and strategic, data, and analytics expertise.
A certified ISO 13485 quality management system ensures that all the tools, procedures, and processes of a project comply with social welfare and medical services regulations, and it helps to guarantee the quality and safety of medical devices.
“Finland has recently updated the national legislation to allow the exploitation of social welfare and healthcare services’ data pools. The national health data accumulated in Finland over decades is a global rarity, and could be a gold mine for Finland if used correctly. Solita Health helps both public and private operators to turn health data into business by introducing the latest agile development methods, data-driven operations, and software expertise into this highly regulated domain”, says Risto Kaikkonen, director of the Solita Health unit. “We work with the customer through the entire product development process from ideas to complete and CE-marked commercial devices.”
An ISO 13485 compliant quality management system is an absolute requirement for the proficient design and production of medical devices and equipment. The standard incorporates the European Union regulations for medical devices and helps manufacturers improve their products by reducing risks and enhancing reliability.
Data-driven services for medical professionals
Solita Health has been the primary product development partner of Duodecim Publishing Company Ltd in the making of the Evidence-Based Medicine Electronic Decision Support system. “Duodecim’s core competence is medical content based on evidence. Our collaboration with Solita Health has allowed us to transform this content into digital services that comply with the industry’s regulations. We are seeking synergies for the development of our digital well-being service business”, says Tuomas Lehto, business manager of integrated services at Duodecim.
Certification clears the way to international markets
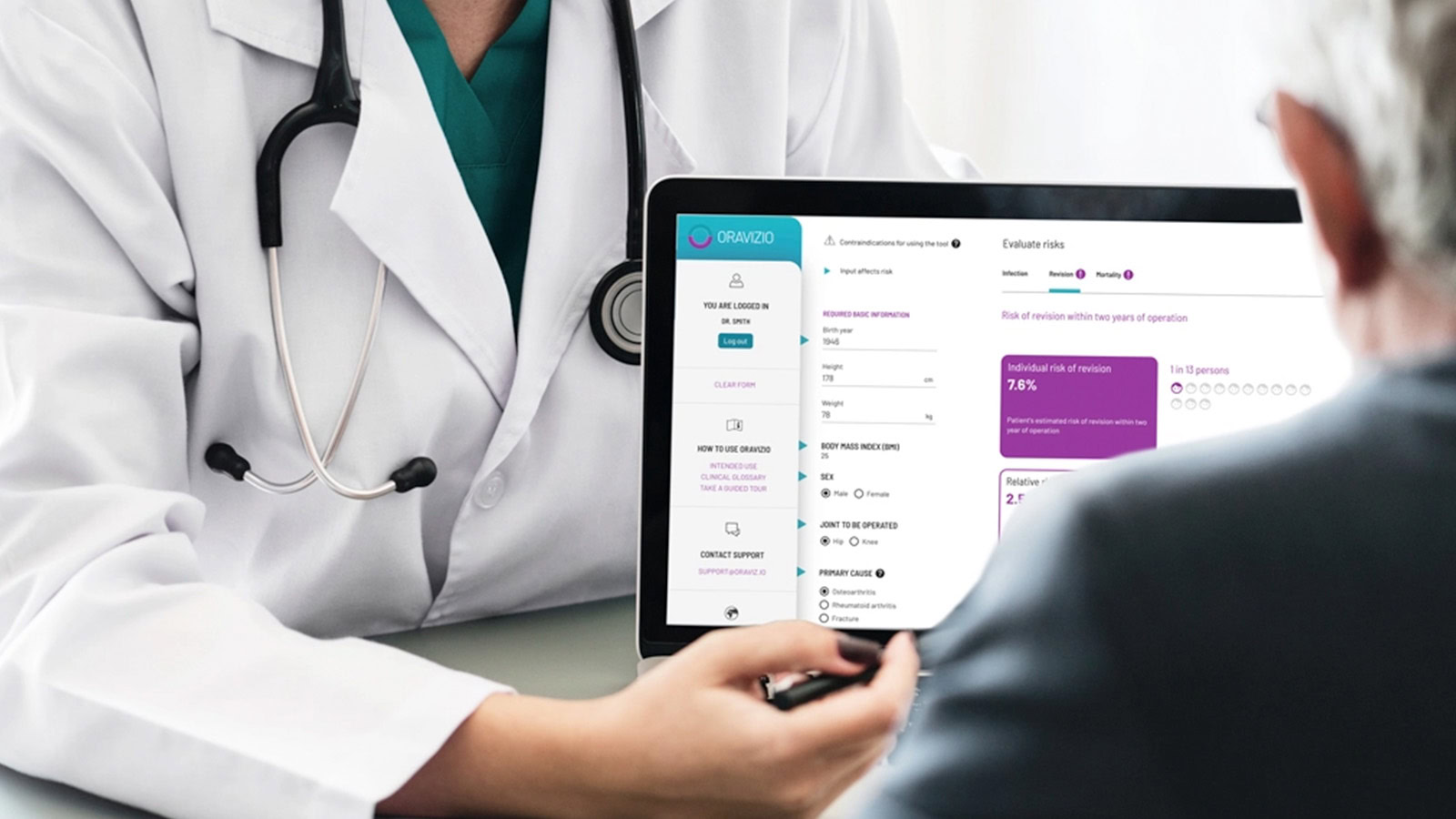
Solita employs more than 100 experts specialised in healthcare, and the company is building digital services for multiple key projects of Finland’s national healthcare system. The current objective is to increase collaboration in product development with medical device manufacturers and export this expertise to international markets. One example of this is the Oravizio risk assessment tool that supports doctors’ decision-making and predicts the risks related to a patient’s surgery.
Further information:
Solita, Risto Kaikkonen, Director, Health and Wellbeing Division, tel. +358 41 536 8745, [email protected]
Quality management system enquiries:
Duodecim Publishing Company Ltd, Tuomas Lehto, Business Manager, tel. +358 50 443 4234, [email protected]
Solita is a fast-growing digital transformation company driven by data and human insight. We create culture, services and tech solutions that help us reinvent businesses and society for the better. Our services range from strategic consulting to service design, digital development, data, AI & analytics and managed cloud services. Established in 1996, Solita now employs close to 1000 digital business specialists in Finland, Sweden, Estonia, Germany, Denmark and Belgium.
Duodecim Publishing Company Ltd publishes information content for medical and healthcare professionals in the form of traditional printed products, databases, solutions integrated into healthcare systems, and as an online learning environment for maintaining professional skills and supporting further training. The content is developed in cooperation with Finland’s leading medical experts. www.duodecim.fi